
Products
Heparin Sodium de Deebio tractans Morbum thromboembolicum praeventionis
Detail
1. Characteres: albi vel fere albi pulveris, valde hygroscopici.
2. Source: porcinum intestinum mucosa.
3. Process: Heparin sodium extrahitur ex mucosa porcino intestino sano.
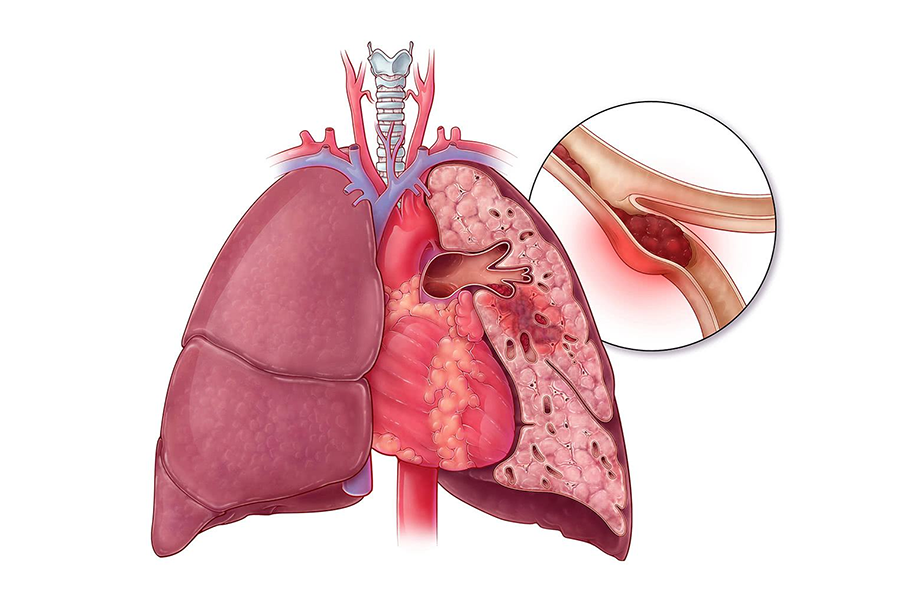
4. Indicationes et usus: Hoc productum praecipue adhibetur ad morbos thromboembolicos praeventionis, praesertim ad necessitatem cito anticoagulantis, ut: 1. Acutus vel chronicus thrombosis venosus vel sanguinis fluxus dynamicae mutationis embolismi pulmonis non significantis (PE) Heparin. embolus extensio sistere potest ad tempus vacandum pro thrombolysi corporis spontanea.2. Praeventionis et curatio fibrillationis atrialis cum embolismo.3. Curatio maturationis diffusae coagulationis intravascularis (DIC).4. Praeventionis et curatio thrombosis arteriarum peripheralium vel myocardialium infarctionis.5. Alia in vitro anticoagulation: sicut chirurgica cardiovascularis, in vitro circulationis, hemodialysis, angiographia, adhiberi potest etiam ad praeparationem speciminis transfusionis vel sanguinis, nunc praecipua indicia applicationis heparin sunt venae altae thrombosis (DVT), PE et thrombosis. in magno periculo aegris.
quare nos?
· Transierunt Sinica GMP
· XXVII annos biologicos enzyme R&D history
· Rudis materia deprauationis
· parere cum USP.EPac elit vexillum
· Export ut supra XXX regiones et regiones
· Facultatem administrationis qualitatis habet ut FDA, Iaponia PMDA, Corea Meridiana MFDS, etc.
Specification
| Test Items | Societas Specification | ||
| EP | USP | ||
| Characteres | Alba vel fere cerussa, altus hygroscopic | ||
| Lepidium sativum | Thrombotest: Conforms | Identitatem chromatographicam: Conforms | |
| 1H NMR Spectrum: Conforms | 1H NMR Spectrum: Conforms | ||
| Liquid Chromatography: Conforms | Pondus mediocris hypothetica pondus: (XV) ~ (XIX) | ||
| Sodium: Conforms | Sodium: Conforms | ||
| Anti-factor Xa ad rationem anti-factorem IIa: 0.9~ 1.1 | Anti-factor Xa ad rationem anti-factorem IIa: 0.9~ 1.1 | ||
| Tests | Claritas et color | Claritas: Serena, Color: per 5 vel melius | ---- |
| Nitrogen | 1.5~2.5%.arida substantia. | 1.3~2.5%.arida substantia. | |
| immunditias nucleotidicas | A260≤ 0.15(4mg/ml) | ≤ 0.1.w/w*. | |
| Substantiae cognatae | Conforms | ---- | |
| Terminus ofgalactosamine in summa hexosamine | ---- | ≤ 1.0% | |
| Oversulfated chondroitin sulfate | ---- | Conforms | |
| pH | 5.5~8.0.1%. | 5.5~7.5.1%. | |
| Damnum in siccitate | ≤ 8.0%.60℃ sicca in Vacuo, 3h. | ≤ 5.0%.60℃ sicca in Vacuo, 3h. | |
| Requiesce in ignitio | ---- | 28.0%~41.0% | |
| Bacterial endotoxin | ≤ 0.01 IU/International Unitas Heparin | ≤ 0.03 USP U / Unitas Internationalis Heparin | |
| Grave metallum | ≤ 30ppm | ≤ 30ppm | |
| Natrium | 10.5~13.5%.arida substantia. | ---- | |
| Dapibus | ≤ 0.5%.arida substantia. | ≤ 0.1%.Ratio pondus. | |
| Actio | ≥ 180 IU/mg.arida substantia. | ≥ 180 USP U/mg.arida substantia. | |
| Microbial immunditias | TAMC | ≤ 1000cfu/g | ≤ 1000cfu/g |
| TYMC | ≤ 100cfu/g* | ≤ 100cfu/g* | |
| E.coli | Conforms | Conforms | |
| Staphylococcus aureus | Conforms | Conforms | |
| Salmonella | Conforms | Conforms | |





















