
Products
Ursodeoxycholic Acidum (UDCA) de Deebio ad Gallstones tractandum
Detail
1. Characteres: cerussa;inodora.Productum hoc in ethanolo solutum est, in chloroformi insolubile;solutum in acido glaciali acetico, et solutum in sodium hydroxide examinis solutione.
2. Processus: Synthetic.
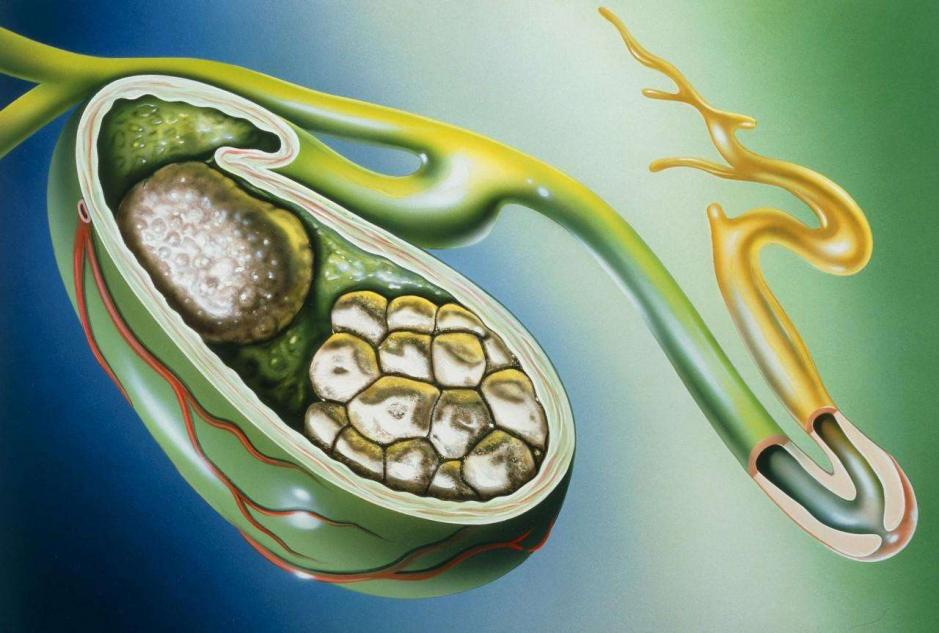
3. Indicationes et usus: Ad curationem gallae, morbo iecoris cholestatici, hepatitis pingue, varias species hepatitis, morbos toxici hepatis, cholecystitis, cholecystitis et dyspepsiae biliariae, bilis refluxus gastritis, etc.
quare nos?
·Productum in officina GMP
· XXVII annos biologicos enzyme R&D history
· Rudis materia deprauationis
· Obtemperate mos ac coeptis vexillum
· Export ut supra XXX regiones et regiones
· Facultatem administrationis qualitatis habet ut FDA, Iaponia PMDA, Corea Meridiana MFDS, etc.
Specification
| Test Items | Secundum internum vexillum ac Customer Standard | |
| APPARENTIA | Pulvis albus vel fere albus. | |
| TABULATUM | Inter CC°C et 205°C | |
| IN SPECIE RATIONE? | +57.0 +62.0° | |
| DE siccitate PRAEIUDICATUM | ≤0.5% | |
| RELICTUM DE IGNIS | ≤0.1% | |
| TEMPTATIO | 98.5% 〜 101.5% (substantia arida) | |
| CASTITATIS (HPLC) | ≥98.5% | |
| Substantia cognata (HPLC) | ≤1.5% (Chenodeoxycholicacid) | Non detectum |
| ≤0.1% (Immunditiae non specificatae) | 0.05% | |
| ≤0.1% (Lithocholicacid) | Non detectum | |
| ≤1.5% (Total) | 0.10% | |
| Substantia cognata (TLC) | ≤O.05% (Acidi lithocholici) | Obsequitur |
| ≤1.5% (Chenodeoxycholicacid) | Obsequitur | |
| RELICTUM SOLVENT | Acetone ≤5000ppm | |
| TOTAL AEROBIC MICROBIAL COUNT | <103cfu/g | |
| TOTAL COMBINED FERMENTUM / fingit COUNT | <102cfu/g | |
| E.COLI | Obsequitur | |
| SALMONELLAE | Obsequitur | |
| CONCLUSIO | secundum quid | |




















